The United States has approved the first anti-chikungunya vaccine, developed by the European Valneva group, health authorities announced in a statement.
The Food and Drug Administration (FDA), whose decisions are widely followed around the world, said that the vaccine, which will be sold under the name “Exchic,” is permitted for people over the age of 18 who are at greater risk of contracting the virus.
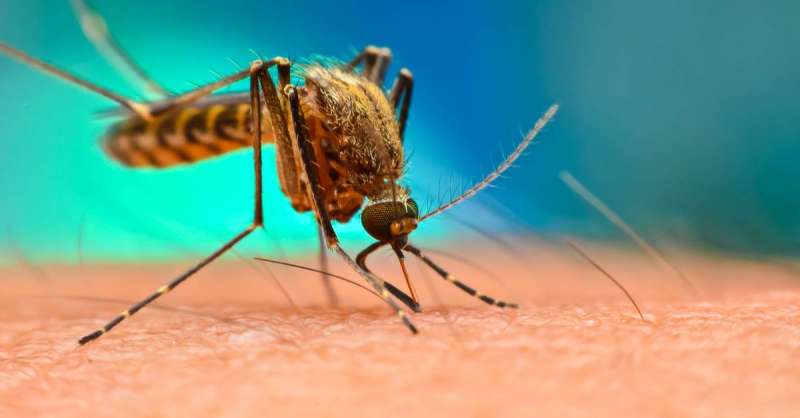
The chikungunya virus, which is transmitted to humans by any mosquito that carries it, causes a sudden onset of fever and severe joint pain.
Symptoms may sometimes last for several months and even years, but deaths resulting from this virus are rare.
This disease is found mainly in tropical regions, specifically in Africa, Southeast Asia, and certain regions of the Americas.
The FDA said chikungunya has spread to other regions, leading to an increase in the number of infections and described the disease as an “emerging global health threat,” and infections have been detected in Europe.
Valneva submitted an application to the European Medicines Agency to obtain approval to sell the vaccine in Europe.
The Food and Drug Administration indicates that at least five million cases of chikungunya have been detected during the last fifteen years.
Food and Drug Administration official Peter Marks said in the statement, “Infections with the virus may lead to serious and long-term health problems, especially among the elderly and those with a medical history.”
The virus can be transmitted to infants from their mothers, and it may be fatal to them.
The person receives a single dose of the vaccine that contains the virus in an attenuated form, a traditional technique used in other vaccines.
Two clinical trials were conducted in North America on several thousand people. The main side effects were headache, fatigue, muscle pain and nausea.