The US Food and Drug Administration (FDA) on Friday approved one of the first effective drugs for Alzheimer’s disease, Lecanemab, which appears to be succeeding in slowing the memory-robbing disease, despite controversy among some experts about its safety and its effectiveness.
The two American companies, “Esay” and “Biogen”, had sought rapid approval from the Food and Drug Administration for the new drug, which works to inhibit amyloid beta, known as “beta amyloid peptide”.
As the new drug is sold under the trade name “Leqembi”, its price is $26,500 (about 8,100 dinars) annually for its semi-weekly doses (675 dinars per month).
A report published by USA Today indicated that this treatment is intended for patients who suffer from a mild degree of Alzheimer’s disease or who are in the early stages of the disease, and they are the ones who have conducted clinical trials on them.
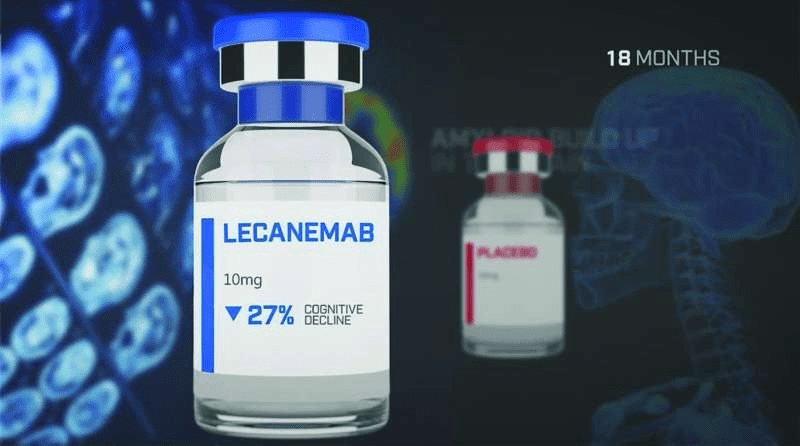
In phase 3 clinical trials, the drug reduced mental decline by 27 percent, but its side effects included swelling and cerebral hemorrhage.
The latest phase study was the latest to test a three-decade-old theory that Alzheimer’s disease is caused by amyloid that builds up in patients’ brains and can be slowed by drugs that remove the buildup of this protein. The new drug works by binding to and neutralizing protofibril groups of amyloid-beta in the brain.
And unlike previous drugs targeting amyloid beta, which failed in large studies, Lecanemab was shown to be effective in slowing cognitive decline in a late-stage clinical study of 1,795 people who received either the drug or a placebo.
“Lecanemab reduced beta-amyloid levels in early-onset Alzheimer’s patients and led to moderate decreases in measures of cognition and function compared to placebo over an 18-month period, but resulted in adverse events,” the researchers who conducted the study wrote in their paper published in the New England Journal of Medicine. .
The researchers noted that longer trials are needed to determine the efficacy and safety of the drug in treating early-onset Alzheimer’s disease.
For his part, said Dr. Billy Dean, director of the Office of Neuroscience in the Center for Drug Evaluation and Research of the Food and Drug Administration, “Alzheimer’s disease is disruptive to the lives of people with it and it has devastating effects. This new treatment option is the latest treatment to target and influence the underlying disease mechanism, rather than just treating the symptoms of the disease.”